| |
You are here: MIT OpenLabWare » Optical Trapping
» Glossary
Glossary
Words are listed alphabetically. Click on a letter to jump to the first word starting with that letter. [A] [B] [C] [D] [E] [F] [G] [H] [I] [K] [L] [M] [N] [O] [P] [R] [S] [T] [U] [V] [W]
acetal
An acetal is a chemical structure with the general structure shown in the figure.
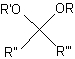
The general structure of an acetal.
Link: Much more information on hemiacetals and acetals.
See also: hemiacetal
acetylation
The process of introducing an acetyl group into a compound. (Dictionary.com)
Link: Acetylation on Wikipedia.
Acoustic Optic Reflectors (AOD)
"A part of the optical trap equipment that ""chops
adenine
One of the four nitrogenous bases that make up DNA. Adenine is a purine.
Link: A brief introduction to the structure of DNA
Alexa488
A water soluble fluorescent dye used for protein and nucleic acid labeling. It is excited at a wavelength of 488 nm.
Link: Alexa488 on Wikipedia.
Alexa555
A water soluble fluorescent dye used for protein and nucleic acid labeling. It is excited at a wavelength of 555 nm.
Link: Alexa555 on Wikipedia.
aliquot
A small portion. It is common practice to subdivide a precious solution of reagent into aliquots that are used when needed without handling the total sample. (CancerWEB)
A known amount of a homogeneous material, assumed to be taken with negligible sampling error. The term is usually applied to fluids. The term "aliquot" is usually used when the fractional part is an exact divisor of the whole; the term "aliquant" has been used when the fractional part is not an exact divisor of the whole (e.g. a 15 mL portion is an aliquant of 100 mL). When a laboratory sample or test sample is aliquoted or otherwise subdivided, the portions have been called split samples. (IUPAC Compendium of Chemical Terminology)
amide
Any compound with the moiety RCONHR.
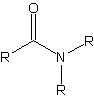
The general structure of amides.
Link: Amide on Wikipedia.
Antibody
Any of a large variety of proteins normally present in the body or produced in response to an antigen which it neutralizes, thus producing an immune response
aromatic
See also: aromaticity
aromaticity
Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. (Wikipedia.org)
Link: Aromaticity on Wikipedia.
bathochromic shift
Shift of a spectral band to lower frequencies (longer wavelengths) owing to the influence of substitution or a change in environment. It is informally referred to as a red shift and is opposite to hypsochromic shift (blue shift). (IUPAC Compendium of Chemical Terminology)
Link: Bathochromic shift on Wikipedia.
Beam Waist
The narrowest point of the laser beam in an optical trap
benzene
A colorless liquid hydrocarbon; highly inflammable; carcinogenic; the simplest of the aromatic compounds. (Dictionary.com)
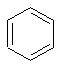
The structure of benzene.
Link: Benzene on Wikipedia.
benzonitrile
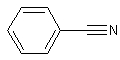
The structure of benzonitrile.
Link: Benzonitrile on Wikipedia.
Biotin
A crystalline, water-soluble vitamin, C10H16O3N2S, of the vitamin B complex, that is present in all living cells and functions as a growth factor and as a catalyst in carboxylation reactions.
bovine
Of, relating to, or resembling a ruminant mammal of the genus Bos, such as an ox, cow, or buffalo. (Dictionary.com)
bronchial
See also: bronchus
bronchus
Either of two main branches of the trachea, leading directly to the lungs. (Dictionary.com)
C-terminal end
The C-terminal end of a protein or polypeptide is the extremity of the amino acid chain terminated by a free carboxyl group (-COOH). Each amino acid has a carboxyl group and an amine group, and amino acids link to one another to form a chain by a dehydration reaction by joining the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an amine group, the N-terminus.
Link: Wikipedia.org
Caged compounds
Compounds that release the effector species generally on a millisecond or faster time scale, upon flash photolysis with near-UV light. They are used principally in studies of rapid biological processes to enable the application of a bioeffector species at or near its site of action.
Link: National Institute for Medical Research
carcinogenesis
The generation of cancer from normal cells, correctly the formation of a carcinoma from epithelial cells, but often used synonymously with transformation, tumourigenesis. (CancerWEB)
Characteristic Modulating Frequency
For a trap that is being modulated, being turned on and off, the characteristic modulating frequency achieves 63% of the trap stiffness possible for a continuous trap of the same power.
chloroform
A clear, colorless, heavy, sweet-smelling liquid, CHCl3, used in refrigerants, propellants, and resins, as a solvent, and sometimes as an anesthetic. Chloroform, once widely used in human and veterinary surgery, has generally been replaced by less toxic, more easily controlled agents. (Dictionary.com)
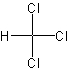
The structure of chloroform.
Link: Chloroform on Wikipedia.
cis
Prefix meaning on this side, on the near side; opposite of trans-. (CancerWEB)
Link: Cis on Wikipedia.
cleavage
The act of splitting or cleaving. (Dictionary.com)
Codon
A triplet of adjacent nucleotides in the messenger RNA chain that codes for a specific amino acid in the synthesis of a protein molecule.
Link: Dictionary.com
Condenser Lens
The part of the microscope that focuses light on the object in question
Corner Frequency
The ratio of the stiffness of the trap to the viscous drag coefficient of the trapped bead.
covalent
Of or relating to a chemical bond characterized by one or more pairs of shared electrons. (Dictionary.com)
Link: Covalent on Wikipedia.
Link: An overview of chemical bonding.
covalently
See also: covalent
crystalline
Being, relating to, or composed of crystal or crystals. (Dictionary.com)
Cy3
A water soluble fluorescent dye used for protein and nucleic acid labeling, part of the cyanine dye family. (Wikipedia.org)
Cysteine (Cys)
A crystalline amino acid
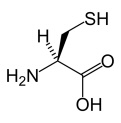
The amino acid L-cysteine.
Link: Dictionary.com
cytosine
One of the four nitrogenous bases that make up DNA. Cytosine is a pyrimidine.
Link: A brief introduction to the structure of DNA
Dalton (Da)
A unit of mass that equals the weight of a hydrogen atom
Link: CancerWEB
Dark box
The part of the optical trap set up that contains the equipment that detects fluorescence
denaturation
To cause the paired strands of (double-stranded DNA) to separate into individual single strands. (Dictionary.com)
To cause the tertiary structure of (a protein) to unfold, as with heat, alkali, or acid, so that some of its original properties, especially its biological activity, are diminished or eliminated. (Dictionary.com)
Link: Denaturation on Wikipedia.
Denature
To induce structural alterations that disrupt the biological activity of a molecule. Often refers to breaking hydrogen bonds between base pairs in double-stranded nucleic acid molecules to produce in single-stranded polynucleotides or altering the secondary and tertiary structure of a protein, destroying its activity.
Link: CancerWEB
denatured
See also: denaturation
deoxyribonucleoside
A nucleoside containing deoxyribose that is a constituent of DNA. (The Free Dictionary)
Link: A brief introduction to the structure of DNA
Derivative
A compound derived or obtained from another and containing essential elements of the parent substance.
Link: Dictionary.com
derivative
A chemical substance derived from another substance either directly or by modification or partial substitution. (CancerWEB)
Link: Derivative on Wikipedia.
See also: derivatization
derivatization
Transformation of a chemical compound into a related one, usually by addition of a functional group.
Link: Derivatization on Wikipedia.
See also: derivative
Dialysis
The process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane.
Link: Wikipedia.org
dichloromethane
A nonflammable liquid CH2Cl2 formerly used as an inhalation anesthetic but now used especially as a solvent, paint remover, and aerosol propellant. (Dictionary.com)
Link: Dichloromethane on Wikipedia.
Dichroic Mirror
Optic that transmits certain wavelengths and transmits others. For example, one of the dichoric mirrors used in the Lang group setup transmits visible light but reflects near-infrared light
Dielectric
A material that conducts electricity poorly or not at all
diethyl ether
A pungent, volatile, highly flammable liquid derived from the distillation of ethyl alcohol with sulfuric acid and widely used as an inhalation anesthetic. (Dictionary.com)
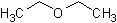
The structure of diethyl ether.
Link: Diethyl ether on Wikipedia.
dihydrofuran
See also: furan
dimethyl formamide (DMF)
Dimethyl formamide is a common laboratory solvent.
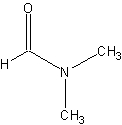
The structure of DMF.
Link: Dimethylformamide on Wikipedia.
dimethyl sulfate (DMS)
Among other things, a methylating agent.
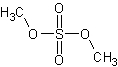
The structure of dimethyl sulfate.
Link: Dimethyl sulfate on Wikipedia.
dimethyl sulfoxide (DMSO)
Dimethyl sulfoxide is a common laboratory solvent.
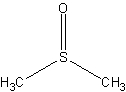
The structure of DMSO.
Link: Dimethylsulfoxide on Wikipedia.
Dipole
A pair of equal and opposite electric charges or magnetic poles separated by a small distance.
DNA
Deoxyribonucleic Acid. This is the genetic material that encodes for proteins and conducts various cellular functions in a living organisms.
Domain
An element of overall structure that is self-stabilizing and often folds independently of the rest of the protein chain. Most domains can be classified into "folds". Domains often are named and singled out because they play an important role in the biological function of the protein they belong to; for example, the "calcium-binding" domain of calmodulin.
Link: Wikipedia.org
Duty Cycle
The fraction of time during which the laser is being operated. It is defined as the ratio of the laser pulse duration to the wave period. The wave period is defined as the amount of time it takes for a full wave to pass a specific point. The pulse duration is the amount of time that the laser is turned on. Therefore, for a laser that has a period of 0.5 microseconds and a pulse duration of 0.25 microseconds, the duty cycle is 50%.
E. coli
A species of rod-shaped, facultatively anaerobic bacteria in the large intestine of humans and other animals, sometimes pathogenic.
Link: Dictionary.com
electrophile
A chemical compound or group that is attracted to electrons and tends to accept electrons.
Link: Electrophile on Wikipedia.
electrophilic
See also: electrophile
emulsion
A suspension of small globules of one liquid in a second liquid with which the first will not mix. (Dictionary.com)
Link: Emulsion on Wikipedia.
epidemiologic
See also: epidemiology
epidemiology
The branch of medicine that deals with the study of the causes, distribution, and control of disease in populations. (Dictionary.com)
Link: Epidemiology on Wikipedia.
epiphase
The upper phase.
Equipartition Theorem
A principle of statistical mechanics. It states that the internal energy of a system composed of a large number of particles at thermal equilibrium will distribute itself evenly among each of the quadratic degrees of freedom allowed to the particles of the system. (wikipedia.org). In their paper, the Lang group used this theorem to determine the stiffness of their optical trap.
ethanol (EtOH)
A colorless volatile flammable liquid, C2H5OH, synthesized or obtained by fermentation of sugars and starches and widely used, either pure or denatured, as a solvent and in drugs, cleaning solutions, explosives, and intoxicating beverages. (Dictionary.com)

The structure of ethanol.
Link: Ethanol on Wikipedia.
etiology
A branch of knowledge concerned with the causes of particular phenomena, specifically a branch of medical science concerned with the causes and origins of diseases. (CancerWEB)
Link: Etiology on Wikipedia.
Eukaryote
An organism with a complex cell or cells
Link: Wikipedia.org
explant
To remove (living tissue) from the natural site of growth and place in a medium for culture. (Dictionary.com)
explants
See also: explant
extraction
A technique for separating components in a mixture that have different solubilities. For example, caffeine can be separated from coffee beans by washing the beans with supercritical fluid carbon dioxide; the caffeine dissolves in the carbon dioxide but flavor compounds do not. Vanillin can be extracted from vanilla beans by shaking the beans with an organic solvent, like ethanol. (General Chemistry Online!, Frostburg State University)
facile
Done or achieved with little effort or difficulty; easy. (Dictionary.com)
Fermentation
A process in which an agent causes an organic substance to break down into simpler substances; especially the anaerobic breakdown of sugar into alcohol.
Link: Dictionary.com
Flow cell
Chamber made of a glass coverslip held across a microscope slide by two strips of double sticky tape. In the Lang group experiments, they hold a volume of 15 microliters.
Fluorescence
Light emitted during absorption of radiation of some other (invisible) wavelength.
Fluorophore
A component of a molecule which allows the molecule to be fluorescent
Fmoc
Stands for (F)luorenyl-(m)eth(o)xy-(c)arbonyl which describes the Fmoc protecting group.
Link: Wikipedia.org
formic acid
A colorless caustic fuming liquid, HCOOH, used in dyeing and finishing textiles and paper and in the manufacture of fumigants, insecticides, and refrigerants. (Dictionary.com)
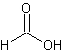
The structure of formic acid.
Link: Formic acid on Wikipedia.
furan
One of a group of colorless, volatile, heterocyclic organic compounds containing a ring of four carbon atoms and one oxygen atom, obtained from wood oils and used in the synthesis of furfural and other organic compounds. (Dictionary.com)
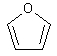
The structure of furan.
Link: Furan on Wikipedia.
gene expression
Conversion of the information encoded in a gene first into messenger RNA and then to a protein. (Dictionary.com)
The full use of the information in a gene via transcription and translation leading to production of a protein and hence the appearance of the phenotype determined by that gene. Gene expression is assumed to be controlled at various points in the sequence leading to protein synthesis and this control is thought to be the major determinant of cellular differentiation in eukaryotes. (CancerWEB)
Link: Gene expression on Wikipedia.
Gene knockout
A genetically engineered organism that carries one or more genes in its chromosomes that have been made inoperative (have been "knocked out" of the organism).
Link: Wikipedia.org
glucose-6-phosphate
An essential intermediate formed from glucose and ATP during the metabolism of glucose. (Dictionary.com)
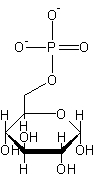
The structure of glucose-6-phosphate.
Link: Glycolysis on Wikipedia.
See also: glucose-6-phosphate dehydrogenase
glucose-6-phosphate dehydrogenase
The pentose phosphate pathway enzyme that uses glucose-6-phosphate to reduce NADP+ to NADPH and H+.
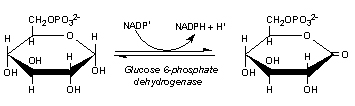
The glucose-6-phosphate dehydrogenase reaction.
Link: Pentose phosphate pathway on Wikipedia.
Link: More detail on the pentose phosphate pathway from RPI.
glycoside
Any compound that contains a carbohydrate molecule (sugar), particularly any such natural product in plants, convertible, by hydrolytic cleavage, into sugar and a nonsugar component (aglycone) and named specifically for the sugar contained, as glucoside (glucose), pentoside (pentose), fructoside (fructose) etc. (CancerWEB)
glycosidic bond
Link: Glycosidic bond on Wikipedia.
See also: glycoside
Green Fluorescent Protein
A protein, comprised of 238 amino acids, from the jellyfish Aequorea victoria that fluoresces green when exposed to blue light.
guanine
One of the four nitrogenous bases that make up DNA. Guanine is a purine.
Link: A brief introduction to the structure of DNA
hemiacetal
A hemiacetal is a chemical structure with the general structure shown in the figure. Hemiacetal means "half acetal" and it refers to the fact that hemiacetals, instead of a second alkoxy (RO) substituent, have a hydroxyl (HO) substituent.
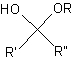
The general structure of a hemiacetal.
Link: Much more information on hemiacetals and acetals.
See also: acetal
hepatocarcinogen
A substance or agent causing cancer of the liver. (Dictionary.com)
hydrocarbon
An organic molecule which consists only of carbon and hydrogen atoms, and no other elements. (CancerWEB)
Link: Hydrocarbon on Wikipedia.
hydrolysate
The product of a hydrolysis reaction. Hydrolysates are generally named according to what reactant was hydrolysed to come up with the product, for example protein hydrolysate if the reactant was a protein. (CancerWEB)
imidazole
Any of a large class of derivatives of imidazole including histidine and histamine. (Dictionary.com)
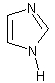
The structure of imidazole.
Link: Imidazole on Wikipedia.
In Phase
Refers to an optical trap condition in which the trapping laser and the excitation laser are on at the same time.
In vitro
(Of a biological process) made to occur in a laboratory vessel or other controlled experimental environment rather than within a living organism or natural setting.
Link: Dictionary.com
in vitro
In an artificial environment outside the living organism. (Dictionary.com)
in vivo
Within a living organism. (Dictionary.com)
intercalation
Intercalation is the "stacking" of a molecule in between the bases of DNA.
Link: Intercalation on Wikipedia.
Interlaced Optical Force-Fluorescence (IOFF)
The method devised by the Lang group in which a material is exposed to alternating pulses of a trapping laser and an excitation laser.
intramolecular
Existing or acting within the molecule. (Dictionary.com)
Irradiation
The act of exposing or the condition of being exposed to radiation.
Link: Dictionary.com
isoamyl alcohol
A primary amyl alcohol that has a disagreeable odor and pungent taste and is obtained from fusel oil. (Dictionary.com)
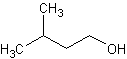
The structure of isoamyl alcohol.
Isotropic
Being independent of direction
Kinase
Any of various enzymes that catalyze the transfer of a phosphate group from a donor, such as ADP or ATP, to an acceptor.
Link: Dictionary.com
Kinesin
A class of motor protein found in cells
Labile
Capable of changing state or becoming inactive when subjected to heat or radiation.
Link: Dictionary.com
lability
Constantly undergoing or likely to undergo change; unstable: a labile compound. (Dictionary.com)
labilization
See also: lability
Laser Head
The source of a laser
lesion
Any pathological or traumatic discontinuity of tissue or loss of function of a part. (CancerWEB)
Link: Lesion on Wikipedia.
Ligation
The act of binding or of applying a ligature.
Link: Dictionary.com
lipophilic
Having an affinity for, tending to combine with, or capable of dissolving in lipids [fats]. (Dictionary.com)
lyophilization
The process of isolating a solid substance from solution by freezing the solution and vaporizing the ice away under vacuum conditions. Also called freeze-drying. (Dictionary.com)
Link: Lyophilization on Wikipedia.
Link: Lyophilization on HowStuffWorks.
Macromolecule
A very large molecule
Link: Dictionary.com
metabolic activation
The metabolic conversion of a parent chemical to another, more potent one.
See also: metabolism
metabolism
The sum of all the physical and chemical processes by which living organised substance is produced and maintained (anabolism) and also the transformation by which energy is made available for the uses of the organism (catabolism). (CancerWEB)
Link: Metabolism on Wikipedia.
metabolite
Any substance produced by metabolism or by a metabolic process. (CancerWEB)
See also: metabolism
methanol (MeOH)
A colorless, toxic, flammable liquid, CH3OH, used as an antifreeze, a general solvent, a fuel, and a denaturant for ethyl alcohol. (Dictionary.com)
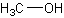
The structure of methanol.
Link: Methanol on Wikipedia.
methylation
Introduction of the methyl group into a chemical compound. (Dictionary.com)
Link: Methylation on Wikipedia.
Microinjection
The process of using a micro needle to insert substances at a microscopic or borderline macroscopic level into a single living cell. It is a simple mechanical process in which an extremely fine micro needle penetrates the cell membrane and sometimes the nuclear envelope and releases its contents.
Link: Wikipedia.org
microsome
Artefactual spherical particle, not present in the living cell, derived from pieces of the endoplasmic reticulum present in homogenates of tissues or cells: microsomes sediment from such homogenates when centrifuged at 106 g and higher: the microsomal fraction obtained in this way is often used as a source of mono-oxygenase enzymes. (IUPAC Compendium of Chemical Terminology)
Modulation Frequency
The instances per second that the laser beam hits the object under investigation
Moiety
One of two or more parts into which something may be divided
Link: Dictionary.com
moiety
Originally, a half; now, loosely, a portion of something. (CancerWEB)
Momentum
In physics, the property or tendency of a moving object to continue moving. For an object moving in a line, the momentum is the mass of the object multiplied by its velocity (linear momentum); thus, a slowly moving, very massive body and a rapidly moving, light body can have the same momentum.
Motility
Ability to move spontaneously and independently
MWCO
Abbreviation for Molecular Weight Cut Off. Dialysis membrane pore sizes are characterized by the molecular weight at which 90% of the solute will be retained (prevented from permeating) by the membrane.
Link: Spectrapor.com
N,N-dimethyl acetamide
N,N-dimethyl acetamide is a common laboratory solvent.
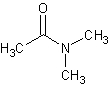
The structure of dimethyl acetamide.
Link: Dimethyl acetamide on Wikipedia.
N-terminal end (N-terminus, N-terminal domain)
Refers to the extremity of a protein or polypeptide terminated by an amino acid with a free amine group (-NH2).
Link: Wikipedia.org
Native chemical ligation
A common form of chemical ligation, a technique for constructing a large peptide from two or more smaller peptides. In native chemical ligation a peptide containing a C-terminal thioester reacts with another peptide containing an N-terminal cysteine, in the presence of an exogenous thiol catalyst. In a thermodynamically-controlled, freely reversible first step a transthioesterification occurs. The product rearranges irreversibly under the usual reaction conditions to form the desired amide bond. The process was developed by Phillip Dawson and Stephen Kent at The Scripps Research Institute in 1994.
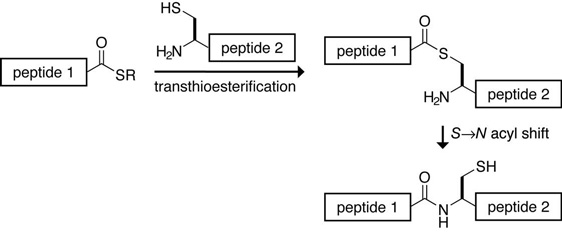
Native chemical ligation.
Link: Wikipedia.org
neoplasm
An abnormal new growth of tissue in animals or plants; a tumor. (Dictionary.com)
neoplastic
See also: neoplasm
Newtons
The SI unit of force, equal to the force that produces an acceleration of one meter per second per second on a mass of one kilogram.
nicotinamide adenine dinucleotide phosphate (NADP)
Nicotinamide adenine dinucleotide phosphate. A coenzyme composed of ribosylnicotinamide 5'-phosphate (nmn) coupled by pyrophosphate linkage to the 5'-phosphate adenosine 2',5'-bisphosphate. It serves as an electron carrier in a number of reactions, being alternately oxidised (NADP+) and reduced (NADPH). (CancerWEB)
Link: NADP on Wikipedia.
nucleic acid
Any of a group of complex compounds found in all living cells and viruses, composed of purines, pyrimidines, carbohydrates, and phosphoric acid. Nucleic acids in the form of DNA and RNA control cellular function and heredity. (Dictionary.com)
Link: Nucleic acid on Wikipedia.
nucleophile
A chemical compound or group that is attracted to nuclei and tends to donate or share electrons.
Link: Nucleophile on Wikipedia.
nucleophilic
See also: nucleophile
nucleoside
Purine or pyrimidine base linked glycosidically to ribose or deoxyribose, but lacking the phosphate residues that would make it a nucleotide. (CancerWEB)
Link: A brief introduction to the structure of DNA
nucleotide
Phosphate esters of nucleosides. The metabolic precursors of nucleic acids are monoesters with phosphate on carbon 5 of the pentose (known as 5' to distinguish sugar from base numbering). (CancerWEB)
Link: A brief introduction to the structure of DNA
Nucleotide
Any of a group of molecules that, when linked together, form the building blocks of DNA or RNA: composed of a phosphate group, the bases adenine, cytosine, guanine, and thymine, and a pentose sugar, in RNA the thymine base being replaced by uracil.
Numerical Aperture
A dimensionless number that characterizes the range of angles over which the system can accept or emit light.
Objective Lens
The part of the microscope in the optical trap that magnifies the specimen and focuses laser beams on it.
Oligonucleotide
A short polymer of two to twenty nucleotides
Optical Tweezer
A device that uses a focus laser beam to trap and manipulate biological molecules in space.
Out of Phase
The condition in an optical trap in which the trapping laser is off when the excitation laser is on and vice versa.
p-nitroperoxybenzoic acid
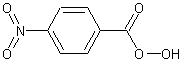
The structure of p-nitroperoxybenzoic acid.
See also: peroxy acid
Peptide
A compound containing two or more amino acids in which the carboxyl group of one acid is linked to the amino group of the other.
Link: Dictionary.com
perchloric acid
A fuming corrosive strong acid HClO4 that is the highest oxyacid of chlorine and a powerful oxidizing agent when heated. (Dictionary.com)
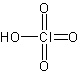
The structure of perchloric acid.
Link: Perchloric acid on Wikipedia.
peroxy acid
Acids with the general structure RCOOH.
Link: Peroxy acid on Wikipedia.
phenobarbital
A crystalline barbiturate used as a sedative, a hypnotic, and an anticonvulsant. (Dictionary.com)
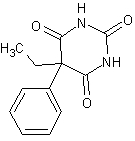
The structure of phenobarbital.
phenol
A caustic, poisonous, white crystalline compound, C6H5OH, derived from benzene and used in resins, plastics, and pharmaceuticals and in dilute form as a disinfectant and antiseptic. Also called carbolic acid. (Dictionary.com)
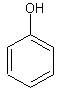
The structure of phenol.
Link: Phenol on Wikipedia.
Phosphate buffered saline
A buffer solution commonly used in biochemistry. It is a salty solution containing sodium chloride, sodium phosphate and potassium phosphate. The buffer helps to maintain a constant pH. The concentration usually matches the human body (isotonic)
Link: Wikipedia.org
Phosphate group
A functional group found in DNA
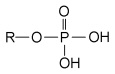
A phosphate group.
Phosphopeptide
A peptide incorporating a phosphate group
Phosphoprotein
A protein which is chemically bonded to a substance containing a phosphate group.
Link: Wikipedia.org
Phosphorylation
The addition of a phosphate group to an organic molecule. Phosphorylation is important for many processes in living cells. ATP is formed during cell respiration from ADP by phosphorylation , as in the mitochondria of eukaryotic cells (oxidative phosphorylation) and the chloroplasts of plant cells (photosynthetic phosphorylation). Phosphorylation also regulates the activity of proteins, such as enzymes, which are often activated by the addition of a phosphate group and deactivated by its removal (called dephosphorylation).
Link: American Heritage Science Dictionary
Photobleach
To lose colour or make white by the action of light; e.g., the use of a laser to bleach a fluorescent dye covalently linked to a macromolecule.
Photolabile
A chemical that is easily dissociated by the absorption of light, usually in the visible or ultraviolet region. An example of a photolabile species is nitrous acid, HONO, which has a very short lifetime in sunlight.
Link: Glossary of Meterology
Photon
The subatomic particle that carries the electromagnetic force and is the quantum of electromagnetic radiation. The photon has a rest mass of zero, but has measurable momentum, exhibits deflection by a gravitational field, and can exert a force. It has no electric charge, has an indefinitely long lifetime, and is its own antiparticle.
phthalate
Salt formed by neutralization of phthalic acid.
See also: phthalic acid
phthalic acid
Phthalic acid is an organic acid.
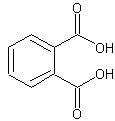
The structure of phthalic acid.
Link: Phthalic acid on Wikipedia.
Piezoelectric stage
The platform on which a microscope slide is placed and can be moved with nanometer precision
polar
Describes a molecule that has a permanent electric dipole. (CancerWEB)
polycyclic
Having more than one cyclic component; especially: having two or more usually fused rings in a molecule. (Dictionary.com)
Polystyrene
A polymer made from the monomer styrene, a liquid hydrocarbon that is commercially manufactured from petroleum
Position Sensitive Device (PSD)
A sensor that can be used to determine the position of a trapped bead relative to its position in the center of the trap with nanometer accuracy
potassium hydroxide
A caustic white solid, KOH, used as a bleach and in the manufacture of soaps, dyes, alkaline batteries, and many potassium compounds. (Dictionary.com)
Link: Potassium hydroxide on Wikipedia.
Proline (Pro)
An alcohol-soluble amino acid
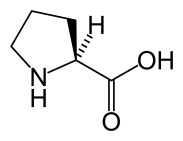
The amino acid L-proline.
Link: Dictionary.com
Pull-down assay
Also known as co-immunoprecipitation. Immunoprecipitation is the technique of precipitating an antigen out of solution using an antibody specific to that antigen. Can identify protein complexes present in cell extracts: by IPing one protein believed to be in a complex, additional members of the complex can also be identified.
Link: Wikipedia.org
purine
A heterocyclic compound with a fused pyrimidine/imidazole ring. Planar and aromatic in character. The parent compound for the purine bases of nucleic acids. (CancerWEB)
Link: Purine on Wikipedia.
putative
Generally regarded as such; supposed. (Dictionary.com)
pyrimidine
A family of 6-membered heterocyclic compounds occurring in nature in a wide variety of forms. They are planar and aromatic in character and include several nucleic acid constituents (cytosine, thymine, and uracil) and form the basic structure of the barbiturates. It is the parent compound of the pyrimidine bases of nucleic acid. (CancerWEB)
Link: Pyrimidine on Wikipedia.
Reconstitute
To constitute again; reconstruct; recompose.
Link: Dictionary.com
recrystallization
Recrystallization is a purification technique that relies on changes in solubility of a compound with changing temperature or solvent composition.
Link: Interactive tutorial on recrystallization from the University of Alberta.
Link: Recrystallization on Wikipedia.
Refractive Force
The force component of the light beam in an optical trap that pushes the targeted object towards the center of the trap.
Refractive Index
The ratio of the velocity of light in a vacuum to that in a medium.
Residue
A single unit within a polymer, such as an amino acid within a polypeptide or protein.
Link: CancerWEB
RNA interference
A mechanism for RNA-guided regulation of gene expression that is common in eukaryotic cells. RNAi involves double-stranded ribonucleic acid (dsRNA) interfering with the expression of genes with sequences that are complementary to this dsRNA.
Link: Wikipedia.org
rotary evaporation
A device used to evaporate solvents.
Link: Rotary evaporation on Wikipedia.
SAPD
Silicone Avalanche Photo Diode. A device that detects photons and measures the fluorescence of the specimen. In the paper it was used to measure the fluorescence decay of the Cy3 dye.
Scattering Force
The force component of the light beam in an optical trap that pushes the targeted object in the direction of light propagation
Schiff base
Any of a class of bases of the general formula R2C=NR that are obtained typically by condensation of an aldehyde or ketone with a primary amine (as aniline) with elimination of water, that usually polymerize readily if made from aliphatic aldehydes, and that are used chiefly as intermediates in organic synthesis and in some cases as dyes Schiff, Hugo Josef (1834-1915), German chemist. In 1864 Schiff discovered the condensation products of aldehydes and amines; the products are now known as Schiff bases. Two years later he introduced a test for aldehydes, in which decolorized fuchsin regains its color in the presence of aldehydes. (Dictionary.com)
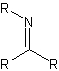
The general structure of Schiff bases (imines).
scintillation counter
A device for detecting and counting scintillations produced by ionizing radiation. (Dictionary.com)
Link: Scintillation counter on Wikipedia.
Secondary structure
The protein structure characterized by folding of the peptide chain into an alpha helix, beta sheet, or random coil.
Link: American Heritage Dictionary
Semisynthesis
Building a complex compound from precursor molecules that are too structurally complex, too costly or too inefficient to be produced by total synthesis.
Link: Wikipedia.org
Site-directed mutagenesis
A molecular biology technique in which a mutation is created at a defined site in a DNA molecule, usually a circular molecule known as a plasmid.
Link: Wikipedia.org
sodium dodecyl sulfate (SDS)
The crystalline sodium salt C12H25NaO4S of sulfated lauryl alcohol; also : a mixture of sulfates of sodium consisting principally of this salt and used as a detergent, wetting, and emulsifying agent (as in toothpastes, ointments, and shampoos). (Dictionary.com)
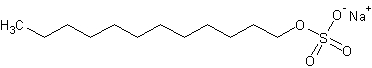
The structure of SDS.
Link: SDS on Wikipedia.
Solid phase peptide synthesis (SPPS)
Process which allows the synthesis of natural peptides which are difficult to express in bacteria, the incorporation of unnatural amino acids, peptide/protein backbone modification, and the synthesis of D-proteins, which consist of D-amino acids. Unlike ribosome protein synthesis, solid-phase peptide synthesis proceeds in a C-terminal to N-terminal fashion. The N-termini of amino acid monomers is protected by these two groups and added onto a deprotected amino acid chain.
Link: Wikipedia.org
Link: SPPS on Sigma-Aldrich
Spectroscopy
The branch of science devoted to discovering the chemical composition of materials by looking at the light (and other kinds of electromagnetic radiation) they emit.
Substrate
The substance acted upon by an enzyme.
Link: Dictionary.com
supernatant
The usually clear liquid overlying material deposited by settling, precipitation, or centrifugation. (Dictionary.com)
TGT resin
A type of resin
Thioester
Compound resulting from the bonding of sulfur with an acyl group with the general formula R-S-CO-R'. They are the product of esterification between a carboxylic acid and a thiol (as opposed to an alcohol in regular esters).
Link: Wikipedia.org
thymine
One of the four nitrogenous bases that make up DNA. Thymine is a pyrimidine.
Link: A brief introduction to the structure of DNA
trans
Prefix denoting across, through, beyond; opposite of cis-. (CancerWEB)
Link: Trans on Wikipedia.
Trap stiffness
A measure of the strength of an optical trap.
Tris
See also: tris(hydroxymethyl)aminomethane
tris(hydroxymethyl)aminomethane (Tris)
A buffer with a pKa of about 8.3 used extensively in biological research.
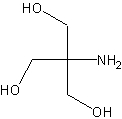
The structure of Tris.
tritium (3H)
Long lived radioactive isotope of hydrogen (half life 12.26 years). Weak emitter, very suitable for autoradiography and relatively easy to incorporate into complex molecules. (CancerWEB)
Link: Tritium on Wikipedia.
Tyrosine (Tyr)
One of the 20 amino acids. Symbol: Y.
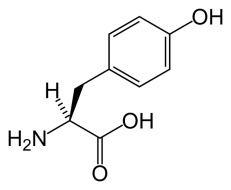
The amino acid L-tyrosine.
Ultraviolet light
Electromagnetic radiation with a wavelength shorter than that of visible light
Link: Wikipedia.org
Variance Method
A way of calculating the stiffness of a trap using equation 3, which is based on the Equipartition Theorem
Waters
Waters Associates is a company that supplies analytical tools to chemists and life scientists.
Link: Waters on the web.
| |