| |
You are here: MIT OpenLabWare » Protein Caging
» Read the Paper
Read the Paper
General Information
This is the HTML version of the paper. Click
here to see a
PDF
file of the paper as it was published in 2007. To view this article at the publisher's website, click here.
Publication Information
Protein Science
Vol. 16, pp. 550-556, March 2007
Semisynthesis of unnatural amino acid mutants of paxillinAbstract
Semisynthesis of unnatural amino acid mutants of paxillin: Protein probes for
cell migration studies
Caged phosphopeptides and phosphoproteins are valuable tools for
dissecting the dynamic role of phosphorylation in complex signaling
networks with temporal and spatial control. Demonstrating the broad
scope of phosphoamino acid caging for studying signaling events, we
report here the semisynthesis of a photolabile precursor to the
cellular migration protein paxillin, which is a complex,
multidomain phosphoprotein. This semisynthetic construct provides
a powerful probe for investigating the influence that
phosphorylation of paxillin at a single site has on cellular
migration. The 61-kDa paxillin construct was assembled using native
chemical ligation to install a caged phosphotyrosine residue at
position 31 of the 557-residue protein, and the probe includes all
other binding and localization determinants in the paxillin
macromolecule, which are essential for creating a native
environment to investigate phosphorylation. Following
semisynthesis, paxillin variants were characterized through
detailed biochemical analyses and by quantitative uncaging
studies.
Keywords: paxillin; native chemical ligation; caging;
phosphoprotein; phosphorylation; semisynthesis
Introduction
The control of cellular adhesion and migration is essential for the
regulation of biological processes, including embryogenesis, wound
repair, and metastasis. Paxillin is a multidomain protein that
orchestrates a pivotal role in these processes by acting as a
dynamic scaffold for signaling and structural proteins. The
phosphorylation of paxillin at specific residues spanning the
macromolecule creates distinct protein-binding sites and thereby
directs paxillin localization to focal adhesions, sites of cell
contact with the extracellular matrix, and influences the
controlled assembly and dissolution of signaling cascades .
For investigating proceses such as cell migration, there is a
need for tools that enable researchers to dissect the dynamic roles
of protein phosphorylation within complex signaling networks.
The essentiality of specific protein phosphorylation events can be
assessed by a number of approaches, including gene knockout, RNA
interference, and site-directed mutagenesis. One limitation with
these strategies is that they cannot afford information on
phosphorylation in "real time." As a complement to these
approaches, the synthesis of caged phosphopeptides, which enable
the controlled release of specific phosphorylated species upon
photolysis, was introduced. Recently, a general method for
the synthesis of these probes has facilitated the application of
caged phosphopeptides in cellular studies . Expanding on this
work, and as part of an initiative to develop generalizable
approaches for the preparation of full-length caged
phosphoproteins, we report the semisynthesis and biochemical
characterization of a mutant of the 61-kDa protein paxillin that
includes a caged phosphotyrosine (cpTyr) residue at position 31
within the N terminus. We similarly report related paxillin
variants with a Tyr or phosphotyrosine (pTyr) at residue 31, which
were constructed through a parallel approach to furnish the
nonphosphorylated and discretely phosphorylated species as
biological controls.
The semisynthesis of the three paxillin variants was accomplished
using native chemical ligation (NCL). NCL is a chemoselective
technique that enables a synthetic peptide to be joined to a
second peptide or a biologically expressed protein fragment
through a native peptide bond. Required for this reaction are a
C-terminal thioester on one moiety and a free cysteine residue at the
N terminus of the second moiety. There are several examples in which
NCL has been applied to create probes for the study of protein
phosphorylation. These include the semisynthesis of
phosphatase-resistant phosphoprotein analogs and a dually caged
phosphoSer-containing derivative of a domain of the protein
Smad2. With the semisynthesis of paxillin analogs, we
demonstrate that NCL can also be applied for the assembly of caged
and phosphorylated variants of full-length proteins, exemplified by
targeting a large, multidomain adaptor protein. Importantly, the
paxillin probe comprises the entire paxillin macromolecule, including
all other binding and localization domains and determinants, which
are essential for creating a native-like system to investigate
phosphorylation at a single site. The approach reported here enables
the semisynthesis of a variety of unique paxillin analogs with
modifications in the N-terminal domain (residues 2-36) in quantities
sufficient for complete biochemical characterization and subsequent
use in biological investigations.
Following semisynthesis, the paxillin variants were characterized
by in vitro binding to a selection of cognate proteins, by
phosphorylation using partner kinases and probed with
phosphoprotein-specific antibodies, and by quantitative uncaging
studies. The semisynthetic caged phosphoTyr31 paxillin permits the
time-sensitive investigation of a single phosphorylation event and
will serve as a valuable tool to probe the role of Tyr31-paxillin
phosphorylation in cellular migration.
Results and Discussions
For the semisynthesis of paxillin (Y31Pax), phosphoTyr31 paxillin
(pY31Pax), and caged phosphoTyr31 paxillin (cpY31Pax), synthetic
thioesters corresponding to residues 2-36 of paxillin were
ligated to a biologically expressed segment comprising paxillin
residues 37-557 (Figure 2). An Asn37Cys mutation was designed in
all constructs to provide a Gly36-Cys37 junction for NCL. This site
was selected because it is in a region of paxillin free from
predicted secondary structure, and because the presence of a Gly as
the terminal thioester residue is known to increase ligation
efficiency.
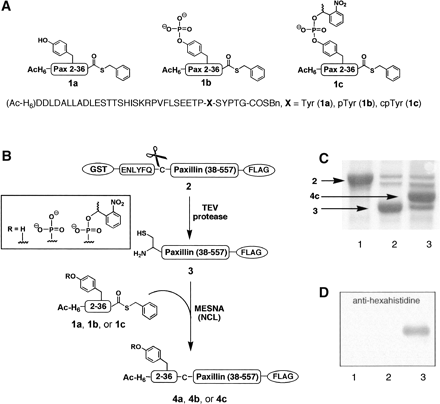
Three peptide thioesters were synthesized corresponding to residues
2-36 of paxillin, and incorporating a Tyr, pTyr, or cpTyr
building block at residue 31. The 41-residue peptides
(Ac-HHHHHH-DDLDALLADLESTTSHISKRPVFLSEETP-X-SYPTG, X = Tyr
[1a], pTyr[1b], or cpTyr [1c]) were prepared by
(fluorenylmethoxy)carbonyl (Fmoc)-based solid-phase peptide synthesis
(SPPS) on the highly acid-labile TGT resin. The peptides were
released from the resin with a C-terminal carboxylic acid and
side-chain protection intact, and were subsequently derivatized to
afford the corresponding C-terminal thioesters. The cpTyr
building block was synthesized as previously described to install a pTyr masked
by the 1-(2-nitrophenyl)ethyl (NPE) caging group. An N-terminal
hexahistidine tag was included in the synthetic peptides to provide a
handle for visualization and purification of ligation products after
NCL.
For the C-terminal fragment of paxillin, residues 37-557 were
expressed as a GST-fusion construct with a FLAG tag (DYKDDDDK)
included at the C terminus to provide a second handle for purification.
A TEV protease cleavage sequence, ENLYFQC, was incorporated
immediately preceding the paxillin insert. TEV protease is a
highly selective cysteine protease that typically recognizes
a serine or glycine in the P1' site, but also will accept a
cysteine residue at that position. Therefore, treatment of
the GST-paxillin(38-557)-FLAG protein (2) with TEV protease
concurrently removed the GST tag and revealed an N-terminal
cysteine residue to afford Cys37-paxillin(38-557)-FLAG (3)
for subsequent ligation. Paxillin is known to be a challenging
protein to express in Escherichia coli, in part because of a
significant number of rare codons, including 27 rare CCC (Pro)
codons. Therefore, to access sufficient quantities of protein
and overcome expression and truncation difficulties, the protein
was fermented in 10-L batches using codon-enhanced cells and
purified via both the N-terminal GST and C-terminal FLAG tags.
The FLAG tag was essential for separating any truncation products
from the full-length protein.
The ligations between 1a, 1b, or 1c and 3 were
conducted in nondenaturing conditions to access Y31Pax (4a),
pY31Pax (4b), and cpY31Pax (4c) in multimilligram
quantities. Exchange of the semisynthetic proteins into PBS using
50-kDa MWCO dialysis membrane concurrently removed unreacted
peptide thioester (MWt 4.8-5.1 kDa).
In vitro characterization of semisynthetic paxillin
Since paxillin is a molecular adaptor protein with no known
enzymatic activity, the function of the reconstituted paxillin
was validated in vitro by analyzing binding to known
paxillin-binding partners and by assessing activity with kinases
that are documented to phosphorylate paxillin. The paxillin-binding
partners focal adhesion kinase (FAK), GRK interactor 1
(GIT1), and PTP-PEST were selected to probe binding along the
entire length of the protein, while the phosphorylation studies
were focused on the NCL-introduced N terminus, which includes the
Tyr31 site.
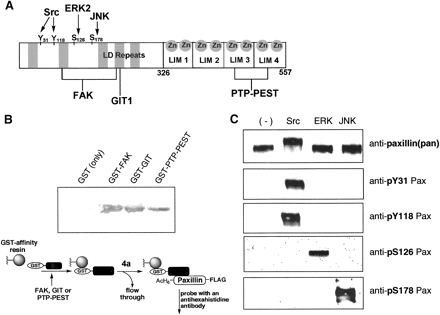
Binding to selected paxillin-interacting proteins
For binding studies, N-terminal GST tags, which are absent in the
ligated paxillin constructs, were expressed with the paxillin-binding
regions of FAK, GIT, and PTP-PEST. The three GST-tagged constructs,
GST-FAK(857-1057), GST-GIT(622-761), and GST-PTP-PEST(338-390),
were evaluated for binding to the semisynthetic Y31Pax (4a)
in a modified GST pull-down assay. The expressed GST-tag alone
(27 kDa) was tested as a negative control to assess nonspecific
interactions. Paxillin binding was detected strongly with the
FAK and GIT constructs, negligibly with GST alone, and weakly
with the PTP-PEST construct. Since PTP-PEST binds at the C-terminal
zinc-binding LIM domains of paxillin, we evaluated whether
improper folding of these domains due to insufficient zinc in the
purified construct contributed to the poor PTP-PEST binding
characteristics. The addition of ZnCl2 to the paxillin
solution significantly increased PTP-PEST binding. Future samples
of paxillin and semisynthetic paxillin derivatives were purified
in the presence of 1 mM ZnCl2. These binding experiments
suggest that the semisynthetic paxillin construct interacts with
known paxillin binding proteins comparably to native paxillin.
Phosphorylation by upstream kinases
Next, to demonstrate that the semisynthetic paxillin analog
functions as a substrate for selected kinases that natively
phosphorylate the protein, phosphorylation of 4a was
attempted with Src, ERK, and JNK and probed with
phosphorylation-specific antibodies to four sites along paxillin.
Src is a tyrosine kinase that is known to phosphorylate paxillin at
Tyr31 and Tyr118, while ERK and JNK are serine/threonine kinases
that phosphorylate residues Ser126 and Ser178, respectively.
In the phosphorylation assays with semisynthetic 4a, residue
Tyr31, a site introduced by ligation, and residue Tyr118 were
phosphorylated exclusively by Src; residue Ser126 was
phosphorylated only by ERK; and residue Ser178 was phosphorylated
only by JNK. The general anti-paxillin antibody recognized protein
in all reactions. Importantly, detection of Src-treated 4a
by the pTyr31 phosphorylation-specific antibody validated both the
successful ligation to install the Tyr31 site and the recognition
of that site by an upstream kinase. As a further control, a
full-length paxillin construct (GST-paxillin[1-557]-FLAG) was
expressed, purified, and subjected to the kinase treatment, and the
expressed paxillin responded identically to the semisynthetic
version (data not shown). The binding and phosphorylation
experiments confirm that the native interactions of the
semisynthetic reconstituted control 4a, including those of
the phosphorylation site of interest, Tyr31, were not compromised
by the expression and ligation procedures.
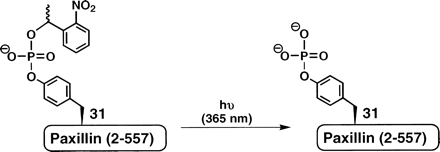
Uncaging of cpY31Pax (4c)
The extent of cpY31Pax (4c) uncaging was quantified following
irradiation with long-wavelength UV light centered at 365 nm.
Importantly for cellular applications, the photo-byproduct of
NPE-caged species, o-nitrosoacetophenone, has previously
been shown to be cellularly inert. This byproduct is
less reactive than the analogous o-nitrosobenzaldehyde
produced by irradiation of widely used
ortho-nitrobenzyl-derived caging groups. For uncaging,
4c was irradiated for 90 sec on a standard DNA
transilluminator. The amount of phosphorylated paxillin (pY31Pax)
present at t = 0 and t = 90 sec post-photolysis was quantified by
chemiluminescent detection of the phosphorylated protein using a
paxillin [pY31] phosphorylation-specific antibody. The total amount
of protein loaded per blot was detected using a general
anti-paxillin antibody. In addition, semisynthetic pY31 (4b)
was used as a photochemically inert internal standard in the
phosphorylation-specific and general paxillin Western blots. The
relative intensity of each sample was determined as a fraction of
total pixel intensity compared to the internal pY31Pax (4b)
standard. The ratio of uncaged phosphopaxillin to the total protein
amount indicated 83%-91% conversion of 4c to
phosphoTyr31-paxillin after irradiation. A minimal amount of
uncaged phosphoprotein, typically <6% of the total protein, was
detected in the absence of intentional uncaging.
Herein, we have described the semisynthesis of paxillin analogs using
an NCL strategy with N-terminal peptide thioesters, allowing access
to multimilligram quantities of cpY31Pax (4c), pY31Pax
(4b), and Y31Pax (4a). Binding and phosphorylation studies
with 4a, including FAK, GIT, and PTP-PEST binding and
phosphorylation by Src, ERK, and JNK, confirmed that the
semisynthetic paxillin derivatives function comparably in vitro to
native paxillin. Uncaging experiments with 4c verify the
applicability of the probe for investigating downstream events of
paxillin Tyr31 phosphorylation. The prevalence of reports of
semisynthetic proteins modified on the C terminus compared to those
modified at the N terminus has been attributed to the challenges of
thioester generation following SPPS. The method used here
is compatible with caged phosphoamino acids and, combined with the
use of an Fmoc-protected caged phosphoTyr amino acid, requires only a
single reaction (thioesterification) beyond standard SPPS protocols.
This design allows the incorporation of a variety of unnatural amino
acid mutations at the N terminus of paxillin.
To date, caged phosphorylated amino acids have been systematically
incorporated into increasingly complex targets, including peptides
and a protein domain. Herein, we have described the culmination
of this progress with the assembly of a full-length, eukaryotic
protein target. Ongoing cellular experiments using microinjected
cpY31Pax (4c) should yield important information on the
effect of Tyr31 phosphorylation on cellular migration.
Materials and Methods
Additional methods are available in Supplemental Methods online.
Thioester synthesis
Peptides were synthesized manually and on an automated (Advanced
ChemTech 396) peptide synthesizer using standard
(fluorenylmethoxy)carbonyl (Fmoc) solid-phase peptide synthesis
(SPPS) protocols on preloaded Fmoc-Gly-Novasyn TGT resin
(Novabiochem). Phosphotyrosine was introduced as
Fmoc-Tyr(PO(OBzl)OH)-OH, and caged phosphotyrosine was introduced
as Fmoc-phospho(1-nitrophenylethyl-2-cyanoethyl)-tyrosine. For
acetylation of the amino terminus of each peptide, 120 μmol of
peptide on resin was treated with acetic anhydride (113 μL, 1.2
mmol) and pyridine (97 μL, 1.2 mmol) in 10 mL of DMF for 30 min.
The peptides were individually cleaved from the resin with
side-chain protection intact by agitating with 0.5% TFA in DCM for
1.5 h. The resin was removed by filtration and rinsed with DCM, the
solvent was mostly evaporated under a stream of nitrogen, and the
peptide was triturated with cold hexanes. The hexanes were removed
in vacuo, and the resulting white powder was dissolved in anhydrous
THF (50 mL) and treated with HBTU (180 mg, 480 μmol), DIPEA (165
μL, 960 μmol), and benzylmercaptan (54 μL, 480 μmol) under
nitrogen overnight. The THF was removed in vacuo and the peptide
was deprotected with 95% (vol/vol) TFA, 2.5% (vol/vol) TIS,
and 2.5% (vol/vol) water for 2 h. Peptides were triturated
with cold diethylether and purified by reverse-phase HPLC on a
Waters 600 instrument with a YMC C18 preparative column
using an elution gradient of water/acetonitrile with 0.1% TFA. The
identities of the peptides as free acids and of the final peptide
thioester products were confirmed by electrospray ionization (ESI)
mass spectrometry on a Perspective Biosystems Mariner
Biospectrometry Workstation (turbo ion source).
Peptide characterization
- Ac-HHHHHHDDLDALLADLESTTSHISKRPVFLSEETP-Y-SYPTG-COOH, Reverse-phase
HPLC (tR = 25.4 min). Exact mass calculated for
C209H307N59O68, 4731.2;
found by MS(ESI), 947.8 [MH5]5+, 790.0
[MH6]6+.
- Ac-HHHHHHDDLDALLADLESTTSHISKRPVFLSEETP-Y-SYPTG-COSBn
(1a), Reverse-phase HPLC (tR = 25.7 min).
Exact mass calculated for
C216H313N59O67S,
4837.3; found by MS(ESI), 968.7 [MH5]5+.
- Ac-HHHHHHDDLDALLADLESTTSHISKRPVFLSEETP-pY-SYPTG-COOH,
Reverse-phase HPLC (tR = 25.4 min). Exact mass
calculated for
C209H308N59O71P,
4811.2; found by MS(ESI), 1204.6 [MH4]4+, 963.9
[MH5]5+.
- Ac-HHHHHHDDLDALLADLESTTSHISKRPVFLSEETP-pY-SYPTG-COSBn
(1b), Reverse-phase HPLC (tR = 25.6 min).
Exact mass calculated for
C216H314N59O70PS, 4917.2;
found by MS(ESI), 1231.9 [MH4]4+, 985.7
[MH5]5+.
- Ac-HHHHHHDDLDALLADLESTTSHISKRPVFLSEETP-cpY-SYPTG-COOH,
Reverse-phase HPLC (tR = 25.4 min). Exact mass
calculated for
C217H315N60O73P,
4960.3; found by MS(ESI), 993.1 [MH5]5+.
- Ac-HHHHHHDDLDALLADLESTTSHISKRPVFLSEETP-cpY-SYPTG-COSBn
(1c), Reverse-phase HPLC (tR = 25.7 min).
Exact mass calculated for
C224H321N60O72PS, 5066.3;
found by MS(ESI), 1014.9 [MH5]5+, 845.9
[MH6]6+.
Plasmid construction for GST-paxillin(38-557)-FLAG
The gene fragment encoding residues 38-557 of paxillin (isoform
 ) was
amplified from a paxillin plasmid (supplied by Martin Schwartz)
with primers to insert 5'-EcoRI and 3'-NotI restriction sites. The
primers also encoded an N-terminal TEV protease recognition site
(ENLYFQC) and a C-terminal FLAG tag. For this amplification the
following PCR primers were used:
5'-GCCGGAATTCGTGAAAACCTGTATTTTCAGTGCCACACATACCAGGAGATT-3' and
5'-GCCCCCTTTTGCGGCCGCCTACTTATCGTCA... ) was
amplified from a paxillin plasmid (supplied by Martin Schwartz)
with primers to insert 5'-EcoRI and 3'-NotI restriction sites. The
primers also encoded an N-terminal TEV protease recognition site
(ENLYFQC) and a C-terminal FLAG tag. For this amplification the
following PCR primers were used:
5'-GCCGGAATTCGTGAAAACCTGTATTTTCAGTGCCACACATACCAGGAGATT-3' and
5'-GCCCCCTTTTGCGGCCGCCTACTTATCGTCA...
....TCGTCTTTGTAGTCGCAGAAGAGCTTGAGGAA-3'.
The PCR-amplified fragments were digested with NotI and EcoRI
and ligated to NotI/EcoRI-digested and CIP-treated pGEX-4T-2
(GE Health Sciences). The ligation mixture was transformed into
DH5 cells and grown on carbenicillin-resistant plates. Plasmid DNA was
isolated from selected colonies and confirmed by sequencing.
cells and grown on carbenicillin-resistant plates. Plasmid DNA was
isolated from selected colonies and confirmed by sequencing.
GST-paxillin(38-557)-FLAG expression and purification
The paxillin plasmid was transformed into BL21-CodonPlus-RP
competent cells (Stratagene) and grown with fermentation at
37°C to midlog phase in 10 L of LB media with carbenicillin
and chloramphenicol. The culture was cooled to 16°C, and the
cells were induced with 0.1 mM IPTG and fermented for 16 h. Cells
were harvested by centrifugation and frozen at -80°C. For cell
lysis, pellets were thawed and resuspended in 350 mL of lysis
buffer (PBS, 1 mM ZnCl2, 1 mg/mL lysozyme, 1 mM DTT, and
Calbiochem protease cocktail III [100 μM AEBSF, 80 nM aprotinin, 5
μM bestatin, 1.5 μM E-64, 2 μM leupeptin, 1 μM pepstatin A]) and
incubated for 20 min at 4°C. The cells were lysed with 1% NP-40
Alternative, then sonicated and subjected to centrifugation at 13,
000 rpm for 30 min, and at 35, 000 rpm for 30 min. The soluble
fraction was purified using 8 mL of Glutathione Sepharose 4 Fast
Flow resin following the manufacturer's protocol. Protein was
dialyzed into TBS and then purified via the carboxy-terminal tag
with 3 mL of anti-FLAG M2 affinity resin (Sigma). Typical
yields for the doubly purified protein were 4-6 mg per 10 L
fermentation, as quantified using a Biorad protein assay. The
purified protein was stored at 4°C and used for all in vitro and
cellular studies within 2 wk of lysis and purification.
TEV protease cleavage
The purified protein 2 was diluted to 0.5 or 1 mg/mL into a
TEV cleavage buffer with a final concentration of 50 mM Tris
pH 8.0, 500 μM EDTA, and 5 mM BME. TEV protease (US Biological)
was added (35 μL of protease per mg of target protein), and
the resulting solution was incubated at 28°C for 3 h. The protein
was dialyzed into TBS (to remove glycine present from the
FLAG-affinity elution) and incubated with Ni/NTA resin and
glutathione sepharose beads to remove the hexahistidine-tagged TEV
protease and the cleaved GST tag. The protein solution was
concentrated using 50-kDa MWCO centrifugal filters (Millipore)
and used immediately in NCL.
Ligations
In general, reactions were carried out with 50 μM protein, 0.8 mM
peptide, and 100 mM MESNA in TBS at pH 8.0. Accordingly, to a
solution of Cys-Pax(38-557)-FLAG (3) (600 μg, 10.7 nmol) in
TBS (150 μL) was added
Ac-HHHHHH-DDLDALLADLESTTSHISKRPVFLSEETP-X-SYPTG-COSBn (lyophilized,
then dissolved into 20 μL of water for transfer; 800 μg, 169 nmol
for X = Tyr [1a], 163 nmol for X = pTyr [1b], and 158
nmol for X = cpTyr [1c]), 10 μL of 2 M MESNA, and 20 μL of
500 mM Tris pH 8.0. The reaction was incubated for 16 h at 25°C,
and then dialyzed into PBS using a 50-kDa MWCO dialysis membrane to
remove excess (4.8-5.1 kDa) peptide. Protein was either used
directly for assays without a final purification or purified using
a Ni/NTA spin column to isolate ligation product via the
ligation-introduced N-terminal hexahistidine tag. The protein was
analyzed by 10% SDS-PAGE gels and visualized with Coomassie blue
dye, and by Western blot with a mouse anti-hexahistidine primary
antibody. For ligations using 1b, a mouse anti-pY31 antibody
was also used for visualization.
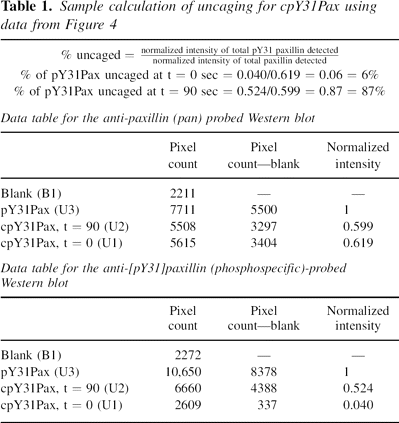
Sample uncaging calculation for cpY31Pax
For uncaging, a 1.2 mg/mL solution of cpY31Pax (4c) in TBS with
2.5 mM DTT was irradiated for 90 sec in a glass cell (pathlength
1 mm) with light centered at 365 nm with an intensity of 7330
μW/cm2 on a DNA transilluminator. Semisynthetic pY31Pax
(4b) and equal amounts of caged and uncaged protein were run
on 10% SDS gels and transferred to nitrocellulose. Western blots
were developed with mouse (monoclonal) anti-human paxillin and
phosphospecific rabbit (polyclonal) anti-paxillin[pY31] primary
antibodies and visualized by chemiluminescence. Pixel counting
was used to calculate the relative intensity, compared to a
photochemically inert standard (4b), of phosphoprotein at t
= 0 and t = 90 sec after uncaging of cpY31Pax (4c). A sample
calculation is shown in Table 1 with data from the Western blot
shown in.
Footnotes
Reprint requests to: Barbara Imperiali, Department of
Chemistry, 77 Massachusetts Ave., 18-590, Massachusetts Institute
of Technology, Cambridge, MA 02139, USA; e-mail:
imper@mit.edu; fax:
(617) 452-2419.
Article published online ahead of print. Article and
publication date are at
http://www.proteinscience.org/cgi/doi/10.1110/ps.062549407.
Supplemental material: see
www.proteinscience.org
Acknowledgements
This research was supported by an NIH Glue grant (GM64346 Cell
Migration Consortium). Support for E.M.V. was provided by the
Merck MIT CSBI fellowship and by the Charles Krakauer Graduate
Fellowship. We thank Professors Rick Horwitz (Univ. of Virginia),
Martin Schwartz (Univ. of Virginia), Tom Parsons (Univ. of
Virginia), and Mike Schaller (Univ. of North Carolina, Chapel Hill)
for the generous donation of GIT, paxillin, FAK, and PTP-PEST
constructs, respectively, and Dr. Erik Schaefer (Invitrogen Corp.)
for the donation of paxillin antibodies.
References
Brown, M.C. and Turner, C.E. 2004. Paxillin: Adapting to
change. Physiol. Rev. 84:
1315-1339.
Dawson, P.E., Muir, T.W., Clark-Lewis, I., and Kent, S.B.H.
1994. Synthesis of proteins by native chemical ligation. Science
266:
776-779.
Futaki, S., Sogawa, K., Maruyama, J., Asahara, T., and Niwa,
M. 1997. Preparation of peptide thioesters using Fmoc-solid-phase peptide
synthesis and its application to the construction of a template-assembled
synthetic protein (TASP). Tetrahedron Lett. 38: 6237-6240.
Hackeng, T.M., Griffin, J.H., and Dawson, P.E. 1999. Protein
synthesis by native chemical ligation: Expanded scope by using straightforward
methodology. Proc. Natl. Acad. Sci. 96:
10068-10073.
Hahn, M.E. and Muir, T.W. 2004. Bioorganic chemistry:
Photocontrol of Smad2, a multiphosphorylated cell-signaling protein, through
caging of activating phosphoserines. Angew. Chem. Int. Ed. Engl.
43:
5800-5803.
Hildebrand, J.D., Schaller, M.D., and Parsons, J.T. 1995.
Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to
the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell
6: 637-647.
Huang, C., Rajfur, Z., Borchers, C., Schaller, M.D., and
Jacobson, K. 2003. JNK phosphorylates paxillin and regulates cell migration.
Nature 424:
219-223.
Humphrey, D., Rajfur, Z., Vazquez, M.E., Scheswohl, D.,
Schaller, M.D., Jacobson, K., and Imperiali, B. 2005. In situ photoactivation
of a caged phosphotyrosine peptide derived from focal adhesion kinase
temporarily halts lamellar extension of single migrating tumor cells. J.
Biol. Chem. 280:
22091-22101.
Kaplan, J.H., Forbush 3rd, B., and Hoffman, J.F. 1978. Rapid
photolytic release of adenosine 5'-triphosphate from a protected analogue:
Utilization by the Na:K pump of human red blood cell ghosts.
Biochemistry 17:
1929-1935.
Lauffenburger, D.A. and Horwitz, A.F. 1996. Cell migration: A
physically integrated molecular process. Cell 84:
359-369.
Lu, W., Shen, K., and Cole, P.A. 2003. Chemical dissection of
the effects of tyrosine phosphorylation of SHP-2. Biochemistry
42:
5461-5468.
Manabe, R.I., Kovalenko, M., Webb, D.J., and Horwitz, A.R.
2002. GIT1 functions in a motile, multi-molecular signaling complex that
regulates protrusive activity and cell migration. J. Cell Sci.
115:
1497-1510.
Mezo, A.R., Cheng, R.P., and Imperiali, B. 2001.
Oligomerization of uniquely folded mini-protein motifs: Development of a
homotrimeric
   peptide. J. Am. Chem. Soc. 123:
3885-3891.
peptide. J. Am. Chem. Soc. 123:
3885-3891.
Muir, T.W.. 2003. Semisynthesis of proteins by expressed
protein ligation. Annu. Rev. Biochem. 72:
249-289.
Nguyen, A., Rothman, D.M., Stehn, J., Imperiali, B., and
Yaffe, M.B. 2004. Caged phosphopeptides reveal a temporal role for 14-3-3 in
G1 arrest and S-phase checkpoint function. Nat. Biotechnol. 22:
993-1000.
Rothman, D.M., Vazquez, M.E., Vogel, E.M., and Imperiali, B.
2002. General method for the synthesis of caged phosphopeptides: Tools for the
exploration of signal transduction pathways. Org. Lett. 4:
2865-2868.
Rothman, D.M., Vazquez, M.E., Vogel, E.M., and Imperiali, B.
2003. Caged phospho-amino acid building blocks for solid-phase peptide
synthesis. J. Org. Chem. 68: 6795-6798.
Schwarzer, D. and Cole, P.A. 2005. Protein semisynthesis and
expressed protein ligation: Chasing a protein's tail. Curr. Opin. Chem.
Biol. 9: 561-569.
Shen, Y., Schneider, G., Cloutier, J.F., Veillette, A., and
Schaller, M.D. 1998. Direct association of protein-tyrosine phosphatase
PTP-PEST with paxillin. J. Biol. Chem. 273:
6474-6481.
Tolbert, T.J. and Wong, C.H. 2002. New methods for proteomic
research: Preparation of proteins with N-terminal cysteines for labeling and
conjugation. Angew. Chem. Int. Ed. Engl. 41:
2171-2174.
Turner, C.E.. 2000. Paxillin and focal adhesion signalling.
Nat. Cell Biol. 2: E231-E236.
Zheng, W., Schwarzer, D., LeBeau, A., Weller, J.L., Klein,
D.C., and Cole, P.A. 2005. Cellular stability of serotonin N-acetyltransferase
conferred by phosphonodifluoromethylene alanine (Pfa) substitution for
Ser-205. J. Biol. Chem. 280:
10462-10467.
| |